There are many different types of watch batteries on the market. It is helpful to have some knowledge of the construction and chemistry of the main types to understand why it is recommended that only silver oxide cells be used as substitutes for mercury cells in tuning fork watches. Don't panic though, we won't be getting into complex chemical formulas here. This is just an overview of the differences between cell types.
We Accutron Lovers not only wish to keep our watches humming and running on time, but want to do this without fear of damaging them. An important feature which distinguishes watch batteries from other button cells (ie. hearing aid batteries) is that they are constructed to very accurate tolerances to minimize the possibility of leaking.
The variation of types available is not simply because battery manufacturers are trying to out-do each other, but because each type of cell has special characteristics making it suitable only for certain applications. Some applications require high-current for short times, some require low current for a long time, some applications require a cheap alternative, some require an extremely constant voltage for the life of the cell. No single type of cell can meet all these requirements, so a variety of different cell constructions and chemistries are used.
A major consideration in battery design and manufacture these days is the environmental consequences related to the disposal of used batteries. This is why the production of mercury batteries has all but stopped. There is only one manufacturer making these cells now (Varta), and they will cease production in 1998.
Click on a link to take you to your area of interest:
Basic Cell Construction

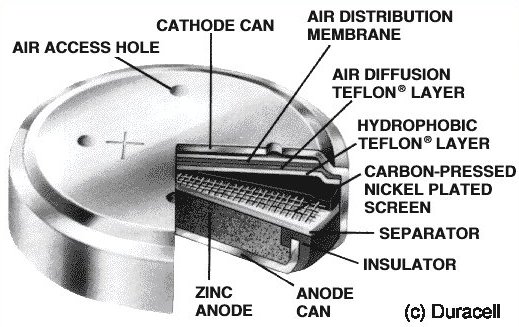
Most button type cells follow the same basic layout in their construction.
1) Anode can - usually containing zinc metal and electrolyte mixture. This forms the negative terminal of the cell.
2) Separator - a porous material containing electrolyte.
3) Cathode can - containing the cathode material/electrolyte mixture. This forms the positive terminal of the cell.
Top
A comparison of the energy stored by different cells on the basis of cell volume:

The amount of energy available from a battery is governed by:
1) The type of cell chemistry used.
2) The amount of chemical material available in the cell.
3) The temperature at which the cell is operated.
The graph show the amounted of energy available from cells of the same volume (size) for a given temperature. Zinc-Air cells clearly have far more energy available. The reasons are given in the section on zinc-air cells below.
Top
A comparison of the voltage discharge charactistics of different cell types:
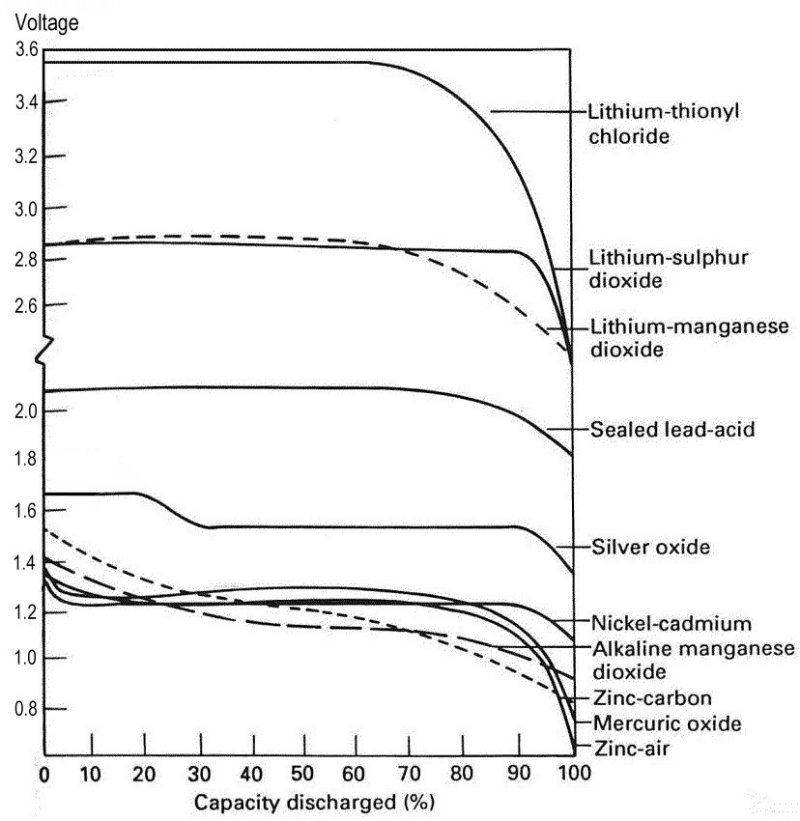
This graph shows the voltage of different cell types over their normal life.
For watches, it is desirable to have a very constant voltage for as long as possible. Watches have a fairly narrow range of operating voltage, and the longer the cell voltage is constant, the higher the amount of total energy stored by the cell is available for use by the watch.
We are interested in Mercury or Silver Oxide batteries for use in our Accutrons.
The working life of these falls in the range:
Silver Oxide - 1.41 to 1.62v
Mercury - 1.05 to 1.36 volts.
Top
Mercury cells
Mercury batteries have been the mainstay of watch batteries for almost 50 years. These cells are rated at 1.36 volts. Due to increasing concerns over waste mercury finding its way into our food, the manufacturing of these batteries has virtually stopped.
Advantages
Extremely constant voltage over its useful life. Suitable for low drain and intermittent high drain applications. Long shelf life - up to 3 years.
Disadvantages
Contains mercury, which in certain forms is highly toxic to humans and animals.
Uses
Excellent for Accutrons, as they were designed originally to operate with these cells. Were used in nearly all applications requiring small constant voltage cells, ie, watches, hearing aids, portable scientific instruments etc.
Construction
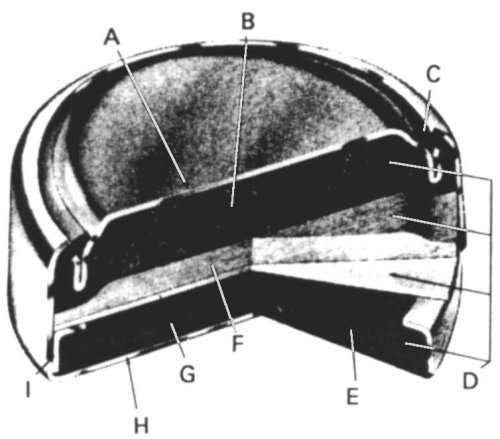
A) Cell top (-ve electrode)
B) Anode of powdered zinc + electrolyte gel
C) Nylon seal
D) Electrolyte
E) Cathode of Mercuric Oxide and Graphite
F) Absorbent separator of fabric and electrolyte
G) Barrier separator membrane.
H) Cell Can (+ve electrode)
I) Metal sleeve to support the nylon case seal
Top
Silver Oxide Cells
The silver oxide cell is really the ideal successor to mercury batteries, and is superior in a number of ways. It has a higher capacity than mercury cells for a given size. These cells are rated at 1.62 volts. Their construction is very similar to mercury cells, the cathode material being the main difference.
Advantages
Constant voltage over its useful life. Contains no chemicals harmful to the environment. Basically superior in all ways over mercury cells.
Disadvantages
About half the shelf life of mercury cells.
Uses
Main applications are in watches. Great for Accutrons, which will perform equally well with these cells as mercury cells.
Construction

A) Cell top (-ve electrode)
B) Anode of powdered zinc + electrolyte gel
C) Nylon seal
D) Electrolyte
E) Cathode of Silver Oxide and Graphite
F) Absorbent separator of fabric and electrolyte
G) Barrier separator membrane.
H) Cell Can (+ve electrode)
I) Metal sleeve to support the nylon case seal
Top
Lithium Cells
Lithium cells are often called "coin cells" due to their shape. The lithium cells used in watches are Lithium-Manganese Dioxide, and are rated at 3.0 volts. The other type of lithium cells commonly seen are Lithium-Thionyl Chloride, which are rated at 3.6 or 3.7 volts. These are not used in watches.
Advantages
Fairly constant voltage over most of its useful life. Contains no chemicals harmful to the environment. Very long shelf life, up to 10 years.
Disadvantages
Suitable for low drain or only intermittent high drain applications.
Uses
Excellent for quartz watches that draw very low current, and only intermittent current when the stepper motor is driven. Can last 5 years or more in the correct application. Not suitable for Accutrons as the voltage is too high.
Construction
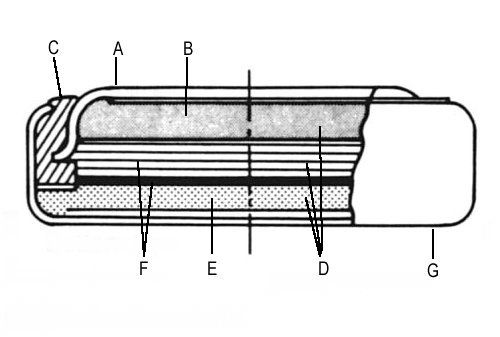
A) Cell top (-ve electrode)
B) Anode of lithium
C) Nylon seal
D) Electrolyte in Separator, Anode and Cathode Material.
E) Cathode of Manganese Dioxide
F) Absorbent separators containing electrolyte
G) Cell Can (+ve electrode)
Top
Alkaline Cells
Alkaline watch batteries are primarily used where cost is a factor. Construction is similar to Lithium cells. Voltage rated at 1.5 volts. Next time you find a free watch in your pack of breakfast cereal, chances are it will be powered by a Zinc Alkaline cell.
Advantages
Cheap to make. Environmentally safe. Good for low and intermittent high drain applications. Will keep that el-cheapo quartz watch going for months!
Disadvantages
Only about half the capacity of a silver oxide cell. Voltage not very constant during its life. Relatively short shelf life, similar to carbon zinc cells in its characteristics, but with about double the stored energy capacity.
Uses
Suitable for applications where a cheap alternative is required. Wouldn't risk putting one in my Accutron even if I could find one to fit.
Construction

A) Cell top (-ve electrode)
B) Anode of Zinc + Electrolyte gel
C) Nylon seal
D) Electrolyte in Separator, Anode and Cathode Material.
E) Cathode of Manganese Dioxide
F) Absorbent separators containing electrolyte
G) Cell Can (+ve electrode)
Top
Zinc-Air Cells
Zinc-Air cells are not watch batteries, and are included in this list because they seem at first glance to be a good alternative to Mercury cells for our Accutrons. Read on and find out why not. Zinc-Air cells have the highest capacity of all button cells due to the fact that most of the cell volume can be taken up with anode material (zinc), because the cathode material used is oxygen obtained from the atmosphere. Their rated voltage is 1.4 volts. They are activated by peeling off an adhesive layer, allowing air to enter the cell through small vent holes.
Advantages
Very high capacity for their size. Very constant voltage ouptut for most of their life. Able to be used in medium current applications. Slightly shorter shelf-life than Mercury cells when not activated, but longer than Alkaline and Silver Oxide. Environmentally safe.
Disadvantages
Must be used in applications where the battery compartment is vented to the atmosphere. The cells are hygroscopic, and therefore may store and release water to and from the atmosphere. Actual performance of the cell can depend on the relative humidity.
Uses
The main use for Zinc Air button cells is in hearing aids. They must not be used in watches, as they require atmospheric oxygen to function, and they may emit water which can be corrosive to metal parts. Under extremes of temperature, or if the cell is shorted out, the internal membranes may rupture, and vent liquids and gas to the atmosphere. Do not use in watches!!!
Construction